What is pH?
- An aqueous solution with a pH = 7.0 has a neutral reaction, which means that H+ and OH- ions are in equilibrium, as in chemically pure water;
- An aqueous solution with a pH < 7.0 has an acidic reaction, meaning that H+ ions are predominant;
- An aqueous solution with a pH > 7.0 has an alkaline reaction, meaning that OH- ions are predominant.
Changes in pH during cultivation
When the results of the substrate solution analyses are interpreted, the pH of the root environment is important, as it affects the availability of nutrients for plants, especially of phosphorus, magnesium, iron and manganese. This physical parameter is extremely variable. During the first phases of production, pH often tends to rise above 7.0. This occurs in response to the uptake of negative ions (anions), e.g. NO3- resulting from strong vegetative growth of plants.
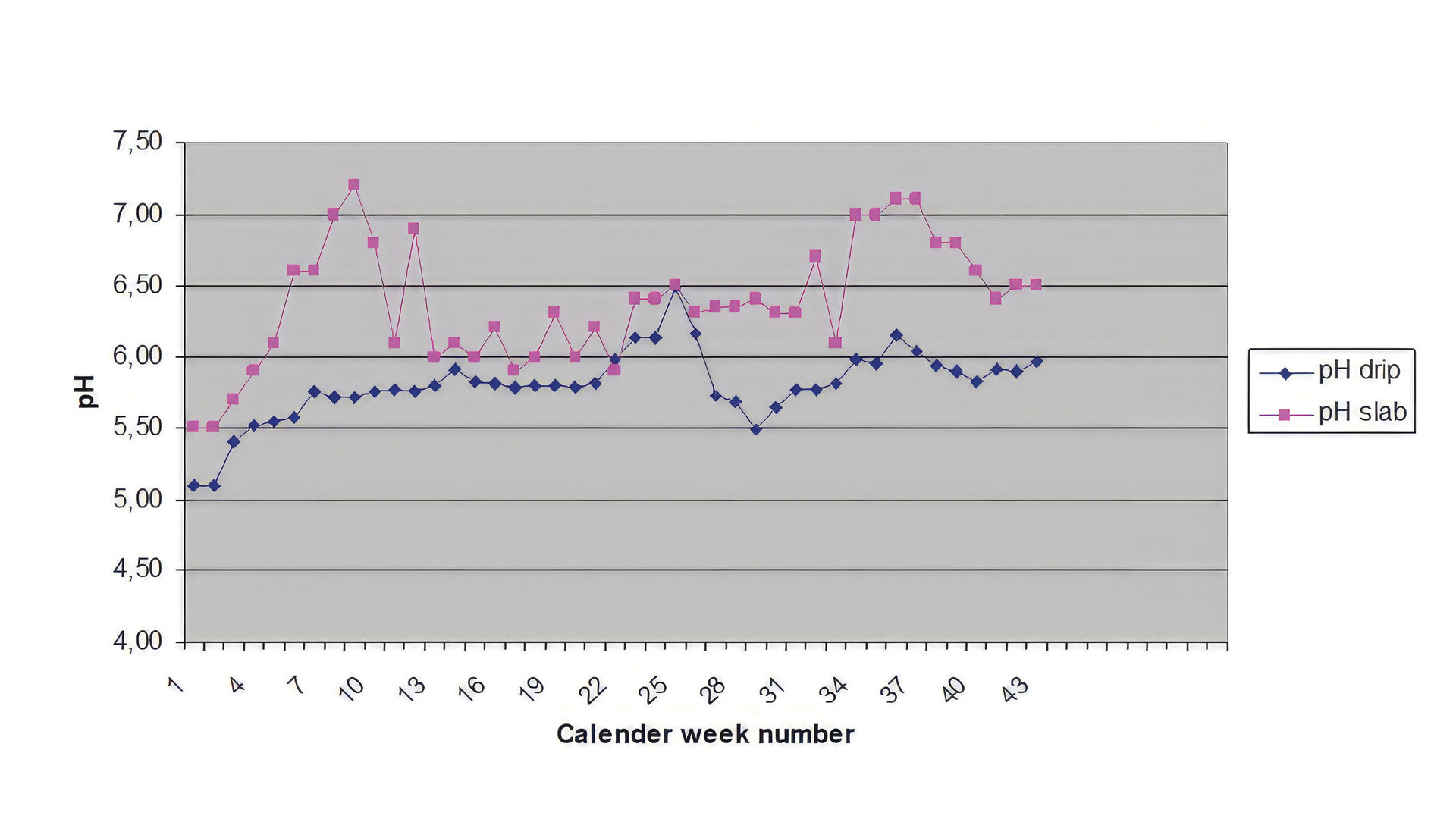
Fig. pH form in the substrate (pHslab Pink) and in the nutrient solution (pHdrip purple) during successive weeks of cultivation
Tips for accurate pH measurement:
- Calibrate the pH meter regularly using standard solutions.
- Check the condition of the batteries, as weak batteries in portable meters are a common cause of errors.
- Store the probe, whenever not in use, in deionised water.
- Store portable meters in a cool and dry place. Do not leave them in the greenhouse.
The main causes of a high pH of the growing medium usually are:
- A high pH of the nutrient solution (> 5.5).
- Strong vegetative growth of the plants.
Actions to correct an excessively high pH:
- Lowering the pH of the nutrient solution to 5.2-5.3 at the dripper.
- Increasing the dose or adding an ammonia form of nitrogen (NH4+-N) ions to the nutrient solution to achieve a level of 1.75 mmol/l (24 ppm). For beef tomatoes, this amount should be a maximum of 1 mmol/l (14 ppm). While adding the NH4+-N ion, always increase the amount of Fe3+, even by 20%.
- Increasing the length of irrigation cycles (with a smaller frequency).
- Controlling the crop so that plant growth is more generative.
- Maintaining an increased fruit load on plants.
The main causes of a low pH of the growing medium usually are:
- A low pH of the nutrient solution fed (< 5.3).
- Incorrect composition of the nutrient solution, i.e. too much ammonium nitrate or ammonium phosphate.
- Generative plant growth with a heavy fruit load.
Actions to correct an excessively low pH:
- Increasing the pH of the nutrient solution to a maximum of 6.0.
- Reducing the amount of NH4+-N in the nutrient solution fed, i.e. < 0.7 mmol/l (10 ppm).
- Reducing the length of irrigation cycles and increasing their frequency.
- Crop control for vegetative growth.
- Maintaining a lower fruit load on plants.
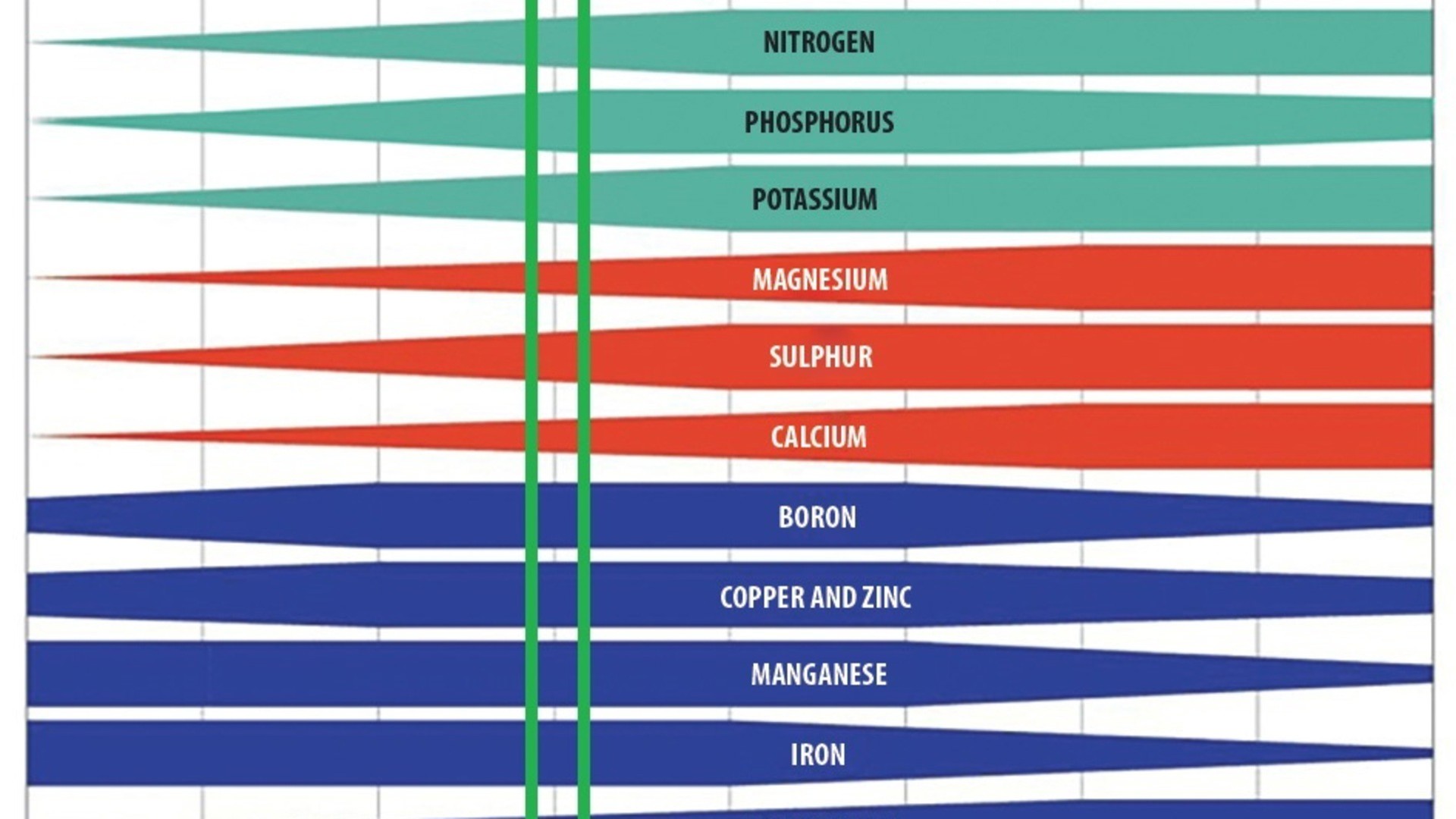
Fig. Macro- and micro-nutrient availability in fertilisers for a given pH of the growing








